Periodic classification Of Elements
The periodic table organizes elements into periods and groups based on their properties and atomic structure. It helps us understand trends and behaviour's of elements, making it a crucial tool in chemistry. The periodic classification of elements refers to the way elements are organized into a table based on their properties. This organization helps us understand the relationships between different elements and predict their behaviour.
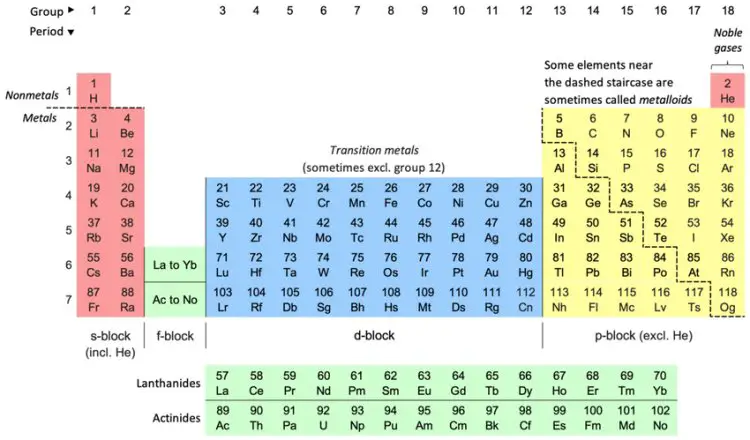
1. What is Periodic Classification?
The periodic classification of elements refers to the way elements are organized into a table based on their properties. This organization helps us understand the relationships between different elements and predict their behavior.
2. The Periodic Table
The periodic table is a chart where all known elements are arranged. Here’s how it’s structured:
-
Periods: These are the horizontal rows in the table. There are 7 periods in total. As you move from left to right across a period, the properties of the elements change gradually.
-
Groups: These are the vertical columns in the table. There are 18 groups. Elements in the same group have similar properties because they have the same number of electrons in their outermost shell.
3. Development of the Periodic Table
-
Dmitri Mendeleev: He is credited with creating the first version of the periodic table in 1869. He arranged the elements based on their atomic masses and observed that elements with similar properties appeared at regular intervals. This pattern is known as periodicity.
-
Modern Periodic Table: Today’s periodic table is organized by atomic number (the number of protons in an atom) rather than atomic mass. This was developed by Moseley in 1913. This arrangement solved some of the problems in Mendeleev’s table and is more accurate.
4. Periodic Properties
-
Atomic Size: Generally, atomic size decreases as you move from left to right across a period and increases as you move down a group.
-
Ionization Energy: This is the energy required to remove an electron from an atom. It generally increases across a period (left to right) and decreases down a group.
-
Electronegativity: This measures an atom's ability to attract and hold onto electrons. It increases across a period and decreases down a group.
5. Classification of Elements
-
Metals, Non-Metals, and Metalloids:
- Metals: They are typically shiny, good conductors of heat and electricity, and are malleable (can be hammered into thin sheets).
- Non-Metals: These are not shiny, poor conductors, and can be gases or solids at room temperature. They often have low melting and boiling points.
- Metalloids: These have properties intermediate between metals and non-metals. They can conduct electricity but are not as good as metals.
-
Alkali Metals: These are in Group 1 and are highly reactive, especially with water. Examples include lithium (Li), sodium (Na), and potassium (K).
-
Alkaline Earth Metals: These are in Group 2 and are less reactive than alkali metals. Examples include magnesium (Mg) and calcium (Ca).
-
Halogens: These are in Group 17 and are very reactive non-metals. Examples include fluorine (F) and chlorine (Cl).
-
Noble Gases: These are in Group 18 and are very unreactive because they have a full outer electron shell. Examples include helium (He) and neon (Ne).
6. The Lanthanides and Actinides
- Lanthanides: These are the elements from lanthanum (La) to lutetium (Lu). They are shiny and have high melting points.
- Actinides: These are the elements from actinium (Ac) to lawrencium (Lr). Many of these are radioactive.
7. Importance of the Periodic Table
The periodic table helps scientists understand the relationships between elements, predict their behavior, and discover new elements. It is a fundamental tool in chemistry and other sciences.
Newlands' Law Of Octaves
Newlands' Law of Octaves, proposed by the English chemist John Newlands in 1864, was an early attempt to classify the chemical elements based on their atomic masses and properties. It’s called the “Law of Octaves” because Newlands noticed that every eighth element seemed to exhibit similar properties, much like the octaves in music.
Here’s a summary of the key points:
-
Element Arrangement: Newlands arranged the known elements in order of increasing atomic mass. He then noticed that elements with similar properties appeared at regular intervals, specifically every eight elements.
-
Octave Analogy: This pattern resembled the musical octave, where the eighth note is similar to the first note of the next octave. For example, if you start with hydrogen and count eight elements to the right, you get to an element with similar properties to hydrogen.
-
Periodic Repetition: Newlands’ observation was an early indication of periodic behavior in elements, a concept that was later formalized into the Periodic Law by Dmitri Mendeleev and others.
-
Limitations: Newlands' Law of Octaves had limitations. It worked well with lighter elements but failed with heavier elements, as the properties of elements did not always repeat every eight elements. Additionally, it didn't account for the existence of noble gases (which were discovered later) or isotopes.
-
Historical Impact: Despite its limitations, Newlands’ work was significant because it demonstrated that there was a periodic relationship between the properties of elements. It paved the way for the development of the modern periodic table.
Newlands' Law of Octaves was one of the earliest attempts to systematize the elements and was a precursor to the more comprehensive periodic tables developed later.
Dmitri Mendeleev's Periodic Table
- Dmitri Mendeleev's periodic table, first published in 1869, was a groundbreaking advancement in chemistry that organized elements based on their atomic masses and properties. Here’s an overview of its key features and significance:
Key Features of Mendeleev’s Periodic Table
-
Arrangement by Atomic Mass: Mendeleev arranged the elements in order of increasing atomic mass. He grouped elements with similar chemical properties into vertical columns, which he called "groups."
-
Periodic Law: Mendeleev’s periodic table was based on the periodic law, which states that the properties of elements are a periodic function of their atomic masses. This means that elements with similar properties recur at regular intervals when arranged by increasing atomic mass.
-
Element Groups: Mendeleev’s table had columns of elements with similar chemical and physical properties, such as alkali metals, alkaline earth metals, halogens, and noble gases.
-
Predictive Power: One of the most remarkable aspects of Mendeleev’s table was its ability to predict the existence and properties of elements that were not yet discovered. He left gaps in the table for these missing elements and predicted their properties with remarkable accuracy.
-
Correction of Atomic Masses: Mendeleev used his table to correct the atomic masses of several elements. For example, he corrected the atomic mass of iodine and told of the presence of an undiscovered element, which he named "eka-silicon" (later discovered as germanium).
-
Periodic Table Layout: The table Mendeleev created had rows (periods) and columns (groups), with elements arranged so that elements in the same row had increasing atomic masses, and elements in the same column had similar chemical properties.
Significance and Impact
-
Foundation for Modern Periodic Table: Mendeleev’s periodic table was the foundation for the modern periodic table. While the initial arrangement was based on atomic mass, later developments, particularly the work of Moseley, shifted the basis of arrangement to atomic number.
-
Discovery of New Elements: The predictions made by Mendeleev for undiscovered elements helped guide future research, leading to the discovery of elements like gallium, scandium, and germanium.
-
Development of Periodic Law: Mendeleev’s work was crucial in establishing the concept of periodicity in chemistry, leading to the development of the modern periodic law, which is based on atomic number rather than atomic mass.
-
Legacy: Mendeleev is often credited with creating the first widely accepted periodic table, which was a major step forward in understanding chemical behavior and relationships among elements.
Mendeleev’s periodic table marked a turning point in chemistry, providing a systematic framework that influenced the study of elements and their interactions. His work laid the groundwork for future advancements in chemistry and the development of the modern periodic table as we know it today.
-
Döbereiner's triads were an early attempt to organize elements based on their chemical properties, introduced by the German chemist Johann Wolfgang Döbereiner in the early 19th century. Here’s a simplified explanation:
Concept of Döbereiner's Triads
-
Groups of Three: Döbereiner noticed that some elements could be grouped into sets of three, which he called "triads."
-
Similar Properties: Elements in each triad had similar chemical properties. For example, the three elements in a triad would react in similar ways with other substances.
-
Average Atomic Mass: Döbereiner observed that the atomic mass of the middle element in each triad was approximately the average of the atomic masses of the other two elements. For instance, if you had a triad of elements A, B, and C, with B being the middle element, then the atomic mass of B was roughly the average of the atomic masses of A and C.
Examples of Döbereiner’s Triads
-
Chlorine, Bromine, and Iodine:
- Chlorine (Cl), Bromine (Br), and Iodine (I) were a triad where the atomic mass of Bromine was roughly the average of Chlorine and Iodine.
-
Calcium, Strontium, and Barium:
- Calcium (Ca), Strontium (Sr), and Barium (Ba) formed another triad, with Strontium’s atomic mass being approximately the average of Calcium and Barium.
Significance
-
Early Classification: Döbereiner’s triads were an important early step in the classification of elements. They demonstrated that elements could be organized based on their properties and atomic masses.
-
Periodic Patterns: While Döbereiner's triads were limited in number and scope, they helped lay the groundwork for the later development of the periodic table, where similar patterns and periodicity were more comprehensively explored by scientists like Mendeleev.
In essence, Döbereiner’s triads were an early effort to group elements with similar properties and showed that atomic masses had a systematic relationship, paving the way for more advanced periodic systems.
-
Summary
The periodic table organizes elements into periods and groups based on their properties and atomic structure. It helps us understand trends and behaviors of elements, making it a crucial tool in chemistry.
What's Your Reaction?





















