symbols of elements, which are shorthand ways to represent elements in chemistry. Here’s a simple guide to understanding these symbols:
What are Element Symbols?
- Definition: Symbols are one or two-letter abbreviations used to represent chemical elements. They make it easier to write and understand chemical formulas and equations.
How Symbols are Formed
- First Letter Capitalized: The first letter of the symbol is always capitalized. For example, O for oxygen.
- Second Letter Lowercase (if needed): If the symbol has a second letter, it is lowercase. For example, Cl for chlorine.
Examples of Element Symbols
- H: Hydrogen
- O: Oxygen
- C: Carbon
- N: Nitrogen
- Na: Sodium (from its Latin name "Natrium")
- Fe: Iron (from its Latin name "Ferrum")
How to Use Element Symbols
- Writing Formulas: Symbols are used to write chemical formulas, which show the types and amounts of atoms in a compound. For example, H₂O stands for water, meaning it has 2 hydrogen atoms and 1 oxygen atom.
- Chemical Equations: Symbols are used in chemical equations to show reactants and products. For example, 2H₂ + O₂ → 2H₂O shows that hydrogen and oxygen react to form water.
Summary in Simple Words
- Symbols are Shortcuts: They are short, easy ways to write the names of elements.
- Capitalization Rule: The first letter is always capitalized; any second letter is lowercase.
- Examples: Hydrogen is H, Oxygen is O, and Iron is Fe.
- Use in Formulas: Symbols are used to write formulas and equations for chemical reactions.
Element symbols are a fundamental part of chemistry, helping you quickly and accurately describe substances and their interactions.
Dalton's Symbols of Elements
-
Historical Context:
- Background: Before the modern system of chemical symbols, John Dalton introduced his own set of symbols in the early 19th century to represent different elements. His symbols were based on simple shapes and letters.
-
Basic Idea:
- Simple Shapes: Dalton used simple shapes like circles, squares, and triangles to represent different elements. Each shape had a different pattern or mark to differentiate between elements.
- Examples:
- Circle (●): Represented an atom of an element.
- Circle with a Dot (•): Sometimes used to represent another element.
- Other Shapes: Squares or triangles with different markings for other elements.
-
Purpose:
- Simplification: These symbols were an early attempt to make it easier to write and understand chemical reactions and compounds.
- Foundation: Dalton’s symbols were a starting point for the development of the modern system of chemical symbols, which are now used universally.
-
Modern Symbols:
- Transition: Dalton's symbols have been replaced by the modern system where each element is represented by one or two letters. For example:
- H for Hydrogen
- O for Oxygen
- C for Carbon
Summary in Simple Words
- Dalton’s Symbols: Early symbols used simple shapes and marks to represent elements.
- Shapes Used: Included circles, squares, and triangles.
- Purpose: Made it easier to write about elements and reactions.
- Modern System: Today, elements are represented by one or two-letter symbols, like H for Hydrogen.
Dalton’s symbols helped pave the way for the more standardized and easily recognizable symbols we use in chemistry today.
Modern Symbols of Elements
-
What They Are:
- Definition: Modern symbols are one or two-letter abbreviations used to represent chemical elements. They make it easy to write and understand chemical formulas and reactions.
-
How They’re Formed:
- First Letter Capitalized: The first letter of the symbol is always capitalized. For example, H for hydrogen.
- Second Letter Lowercase (if needed): If there is a second letter, it is lowercase. For example, Cl for chlorine.
-
Examples of Element Symbols:
- H: Hydrogen
- O: Oxygen
- C: Carbon
- N: Nitrogen
- Na: Sodium (from the Latin name “Natrium”)
- Fe: Iron (from the Latin name “Ferrum”)
- Au: Gold (from the Latin name “Aurum”)
-
Why They’re Useful:
- Simplify Writing: Symbols help simplify the writing of chemical formulas. For example, H₂O represents water, showing it has 2 hydrogen atoms and 1 oxygen atom.
- Chemical Equations: Symbols are used in equations to show how substances react. For example, 2H₂ + O₂ → 2H₂O shows hydrogen and oxygen react to make water.
Summary in Simple Words
- Symbols Are Shortcuts: They are short letters used to represent elements.
- Capitalization: The first letter is always capitalized; the second, if there is one, is lowercase.
- Examples: H for Hydrogen, O for Oxygen, Fe for Iron.
- Usefulness: They make it easier to write chemical names, formulas, and reactions.
Modern element symbols help make chemistry clearer and more straightforward by providing a simple and standardized way to represent each element.
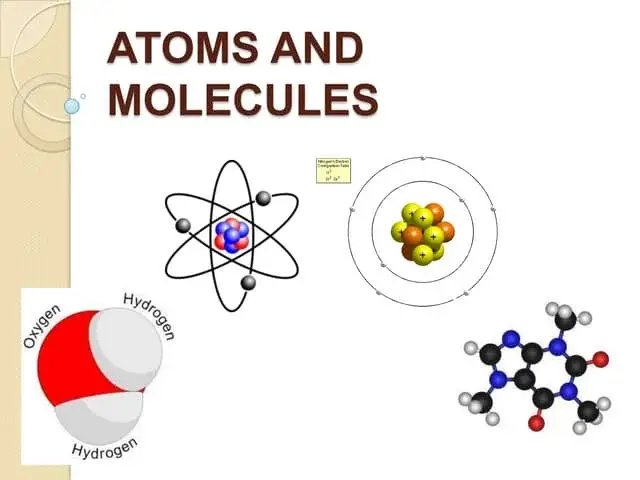
How Do Atoms Exist?
-
Basic Structure:
- Atoms are Tiny Particles: Atoms are the smallest units of matter that still retain the properties of an element.
- Parts of an Atom: Each atom has three main parts:
- Nucleus: The central part of the atom, which contains protons (positively charged) and neutrons (neutral, with no charge).
- Electrons: Tiny, negatively charged particles that orbit around the nucleus in electron shells or energy levels.
-
Electron Shells:
- Electron Arrangement: Electrons are arranged in shells or energy levels around the nucleus. These shells are like layers of an onion.
- Shells Hold Electrons: Each shell can hold a certain number of electrons. For example, the first shell can hold up to 2 electrons, while the second shell can hold up to 8 electrons.
-
Chemical Bonds:
- Atoms Bond Together: Atoms can join together to form molecules through chemical bonds. There are two main types of bonds:
- Covalent Bonds: Atoms share electrons to fill their outer shells. For example, in a water molecule (H₂O), hydrogen and oxygen share electrons.
- Ionic Bonds: Atoms transfer electrons from one to another. For example, in table salt (NaCl), sodium gives an electron to chlorine.
-
Stable Atoms:
- Full Outer Shells: Atoms are more stable when their outer electron shells are full. This often makes them bond with other atoms to achieve a full outer shell.
Summary in Simple Words
- Tiny Particles: Atoms are very small and are the basic units of all matter.
- Parts of Atoms: Each atom has a nucleus (with protons and neutrons) and electrons that orbit around it.
- Electron Shells: Electrons are arranged in shells around the nucleus.
- Bonds: Atoms can stick together by sharing or transferring electrons to form molecules.
- Stability: Atoms are more stable when their outer electron shells are full.
Understanding how atoms exist and bond helps explain how different substances are formed and how they interact with each other.
Molecules
-
What They Are:
- Definition: Molecules are groups of two or more atoms that are chemically bonded together. They are the smallest unit of a substance that can still retain its chemical properties.
- Examples: Water (H₂O) is a molecule made of two hydrogen atoms and one oxygen atom bonded together. Carbon dioxide (CO₂) is a molecule with one carbon atom and two oxygen atoms.
-
Types of Bonds:
- Covalent Bonds: Atoms in a molecule share electrons to stay together. For example, in a water molecule (H₂O), hydrogen and oxygen share electrons.
Ions
-
What They Are:
- Definition: Ions are charged particles that form when atoms gain or lose electrons. This gives them an electric charge.
- Types:
- Cations: Positively charged ions that form when an atom loses one or more electrons. For example, sodium (Na⁺) loses an electron to become positively charged.
- Anions: Negatively charged ions that form when an atom gains one or more electrons. For example, chloride (Cl⁻) gains an electron to become negatively charged.
-
Formation of Ions:
- Loss of Electrons: An atom that loses electrons becomes a cation (e.g., Na → Na⁺ + e⁻).
- Gain of Electrons: An atom that gains electrons becomes an anion (e.g., Cl + e⁻ → Cl⁻).
-
Ionic Bonds:
- Formation: Ions can bond together to form ionic compounds. Positive and negative ions attract each other and stick together. For example, sodium chloride (NaCl) is formed when sodium (Na⁺) and chloride (Cl⁻) ions bond.
Summary in Simple Words
- Molecules: Groups of atoms bonded together, like H₂O (water) or CO₂ (carbon dioxide).
- Ions: Charged particles that form when atoms gain or lose electrons.
- Cations: Positive ions (lose electrons).
- Anions: Negative ions (gain electrons).
- Ionic Bonds: Form when positive and negative ions attract each other to make compounds, like table salt (NaCl).
Understanding molecules and ions helps explain how different substances are formed and how they interact in chemical reactions.
What is Valency?
- Definition: Valency is the ability of an atom to combine with other atoms. It is determined by the number of electrons an atom needs to gain, lose, or share to complete its outer electron shell and become stable.
How Valency Works
-
Outer Shell Electrons:
- Stable Atom: Atoms are most stable when their outermost electron shell is full. For most elements, this means having 8 electrons in the outer shell (the octet rule).
- Valency Electrons: The number of electrons an atom needs to gain, lose, or share to complete its outer shell is its valency.
-
Types of Valency:
- Positive Valency: Atoms with a positive valency lose electrons to achieve a full outer shell. For example, sodium (Na) has 1 electron in its outer shell and loses it to become Na⁺ with a valency of +1.
- Negative Valency: Atoms with a negative valency gain electrons to fill their outer shell. For example, chlorine (Cl) has 7 electrons in its outer shell and gains 1 electron to become Cl⁻ with a valency of -1.
- Neutral Valency: Some atoms share electrons rather than gaining or losing them. For example, carbon (C) has 4 electrons in its outer shell and needs to share 4 electrons to achieve stability.
-
Combining Atoms:
- Forming Compounds: Atoms combine in ways that allow them to achieve full outer shells. For example, hydrogen (H) with a valency of +1 combines with oxygen (O) with a valency of -2 to form water (H₂O).
Summary in Simple Words
- Valency: The ability of an atom to bond with other atoms based on the number of electrons it needs to gain, lose, or share.
- Positive Valency: Atoms lose electrons to become stable (e.g., sodium loses 1 electron).
- Negative Valency: Atoms gain electrons to become stable (e.g., chlorine gains 1 electron).
- Combining Atoms: Atoms combine in ways that help them complete their outer electron shells, forming compounds like water (H₂O).
Understanding valency helps explain how different elements interact to form compounds and why they combine in specific ways.
Atomic Mass
-
Definition:
- Atomic Mass: The atomic mass of an atom is a measure of how heavy it is compared to other atoms. It is usually expressed in atomic mass units (amu or u).
-
How It’s Measured:
- Protons and Neutrons: The atomic mass is approximately equal to the total number of protons and neutrons in the atom’s nucleus. Electrons have a very small mass compared to protons and neutrons and are usually not considered in the atomic mass.
-
Examples:
- Hydrogen (H): Has an atomic mass of about 1 amu.
- Carbon (C): Has an atomic mass of about 12 amu.
Molecular Mass
-
Definition:
- Molecular Mass: The molecular mass (or molecular weight) is the total mass of all the atoms in a molecule. It is also expressed in atomic mass units (amu or u).
-
How It’s Calculated:
- Adding Atomic Masses: To find the molecular mass, add up the atomic masses of all the atoms in the molecule. For example, in a water molecule (H₂O):
- Hydrogen (H): 2 atoms × 1 amu = 2 amu
- Oxygen (O): 1 atom × 16 amu = 16 amu
- Total Molecular Mass: 2 amu + 16 amu = 18 amu
-
Examples:
- Water (H₂O): Molecular mass is 18 amu.
- Carbon Dioxide (CO₂): Molecular mass is calculated as:
- Carbon (C): 1 atom × 12 amu = 12 amu
- Oxygen (O): 2 atoms × 16 amu = 32 amu
- Total Molecular Mass: 12 amu + 32 amu = 44 amu
Summary in Simple Words
- Atomic Mass: The weight of a single atom, measured in atomic mass units (amu), based on the number of protons and neutrons in the atom.
- Molecular Mass: The weight of a molecule, measured by adding up the atomic masses of all the atoms in the molecule.
- Examples:
- Water (H₂O) has a molecular mass of 18 amu (2 for H + 16 for O).
- Carbon Dioxide (CO₂) has a molecular mass of 44 amu (12 for C + 32 for O).
Understanding these measurements helps in knowing how heavy different substances are and how they combine to form new substances.
Composition of a Compound
-
What It Means:
- Definition: The composition of a compound refers to the types and amounts of elements that make up the compound. It tells us which elements are present and how they are combined.
-
Chemical Formula:
- Formula: A compound’s composition is shown using a chemical formula. This formula uses symbols for the elements and numbers to show how many atoms of each element are in the compound.
- Examples:
- Water (H₂O): The formula means each water molecule has 2 hydrogen (H) atoms and 1 oxygen (O) atom.
- Carbon Dioxide (CO₂): The formula means each carbon dioxide molecule has 1 carbon (C) atom and 2 oxygen (O) atoms.
-
Understanding Ratios:
- Proportions: The numbers in a formula show the ratio of atoms of each element in the compound. For example:
- In H₂O: The ratio of hydrogen to oxygen is 2:1.
- In CO₂: The ratio of carbon to oxygen is 1:2.
-
Molecular Composition:
- Molecular Example: If you look at a molecule of glucose (C₆H₁₂O₆), it is composed of:
- 6 Carbon (C) atoms
- 12 Hydrogen (H) atoms
- 6 Oxygen (O) atoms
Summary in Simple Words
- Composition: Tells us what elements are in a compound and how many of each element there are.
- Chemical Formula: Shows this composition using symbols and numbers. For example:
- Water (H₂O): 2 hydrogen atoms and 1 oxygen atom.
- Carbon Dioxide (CO₂): 1 carbon atom and 2 oxygen atoms.
- Ratios: The formula also tells us the ratio of the different atoms in the compound.
Understanding the composition of a compound helps you know what it is made of and how its different parts fit together.
Mole Concept
-
What is a Mole?
- Definition: A mole is a unit used to count particles, like atoms or molecules. It’s like a “chemist’s dozen” but much larger.
- Number of Particles: One mole contains 6.022 × 10²³ particles. This number is called Avogadro’s number.
-
Why Use Moles?
- Counting Large Numbers: Atoms and molecules are incredibly tiny, so using moles helps us deal with large quantities more easily. Instead of counting individual atoms or molecules, we use moles to measure amounts.
-
Molar Mass:
- Definition: Molar mass is the mass of one mole of a substance, measured in grams per mole (g/mol). It’s the same number as the atomic or molecular mass but expressed in grams.
- Example:
- Hydrogen (H): Has an atomic mass of about 1 amu, so the molar mass is 1 gram per mole.
- Water (H₂O): The molar mass is the sum of the atomic masses of its atoms (2 × 1 for hydrogen + 16 for oxygen = 18 g/mol).
-
Using Moles:
- Calculations: You can use moles to convert between the number of particles and the mass of a substance.
- Mass to Moles: Divide the mass of the substance by its molar mass.
- Example: If you have 18 grams of water (H₂O), and the molar mass of water is 18 g/mol, then you have 1 mole of water.
- Moles to Particles: Multiply the number of moles by Avogadro’s number to find the number of particles.
Summary in Simple Words
- Mole: A unit to count particles like atoms or molecules, equal to 6.022 × 10²³ particles.
- Molar Mass: The mass of one mole of a substance, measured in grams per mole.
- Why It’s Useful: It helps us work with large numbers of tiny particles in a manageable way.
- Conversions:
- Mass to Moles: Divide the mass by the molar mass.
- Moles to Particles: Multiply the number of moles by Avogadro’s number.
The mole concept helps simplify calculations in chemistry, making it easier to understand and measure different substances.
1. Finding Moles from Mass
Example: How many moles are there in 18 grams of water (H₂O)?
Solution:
-
Find the Molar Mass of Water (H₂O):
- Hydrogen (H) has an atomic mass of 1 amu, and there are 2 hydrogen atoms.
- Oxygen (O) has an atomic mass of 16 amu.
- Molar mass of H₂O = (2 × 1) + 16 = 18 grams per mole.
-
Calculate the Number of Moles:
- Use the formula: Number of Moles=Molar MassMass of the Substance
- For 18 grams of water: Number of Moles=18 g/mol18 grams=1 mole
Answer: There is 1 mole of water in 18 grams.
2. Finding Mass from Moles
Example: What is the mass of 2 moles of sodium chloride (NaCl)?
Solution:
-
Find the Molar Mass of Sodium Chloride (NaCl):
- Sodium (Na) has an atomic mass of 23 amu.
- Chlorine (Cl) has an atomic mass of 35.5 amu.
- Molar mass of NaCl = 23 + 35.5 = 58.5 grams per mole.
-
Calculate the Mass:
- Use the formula: Mass=Number of Moles×Molar Mass
- For 2 moles of NaCl: Mass=2 moles×58.5 g/mol=117 grams
Answer: The mass of 2 moles of sodium chloride is 117 grams.
3. Finding Number of Particles from Moles
Example: How many molecules are there in 0.5 moles of carbon dioxide (CO₂)?
Solution:
- Find the Number of Molecules:
- Use Avogadro’s number: 6.022×1023 molecules per mole.
- Number of molecules = Number of Moles × Avogadro’s Number.
- For 0.5 moles of CO₂: Number of Molecules=0.5 moles×6.022×1023 molecules/mole Number of Molecules=3.011×1023 molecules
Answer: There are 3.011×1023 molecules in 0.5 moles of carbon dioxide.
4. Finding Moles from Number of Particles
Example: How many moles are there in 1.2044×1023 molecules of methane (CH₄)?
Solution:
- Calculate the Number of Moles:
- Use Avogadro’s number: 6.022×1023 molecules per mole.
- Number of moles = Number of Molecules / Avogadro’s Number.
- For 1.2044×1023 molecules of CH₄: Number of Moles=6.022×1023 molecules/mole1.2044×1023 molecules Number of Moles≈0.2 moles
Answer: There are approximately 0.2 moles of methane in 1.2044×1023 molecules.
These examples cover basic calculations involving the mole concept, which will help you understand how to work with moles, masses, and particle counts in chemistry.